NUCLEOSOME
Our lab is dedicated to deciphering the nucleosomal regulatory network formed by histone-modifying enzyme complexes, DNA-modifying enzymes, and chromatin remodeling complexes, aiming to reveal the molecular mechanisms by which these factors mediate nucleosome assembly, modification, and conformational changes.
Regulation of Histone Methylation
The MLL family of methyltransferase proteins is named after the first discovered gene in the family—Mixed Lineage Leukemia 1 (MLL1)—which is frequently rearranged in acute lymphoblastic and myeloid leukemia. In mammals, the MLL family of methyltransferases includes MLL1, MLL2, MLL3, MLL4, SET1A, and SET1B. These MLL proteins play essential and non-redundant roles in regulating gene expression. In contrast to other histone methyltransferases, the intrinsic methyltransferase activities of MLL proteins are extremely low. The optimal activity of MLL proteins requires the formation of complexes with several regulatory subunits. Our lab’s research has revealed the molecular mechanisms by which these regulatory subunits (WDR5, RBBP5, ASH2L, and DPY30) bind MLLs and regulate the enzymatic activity of MLLs.
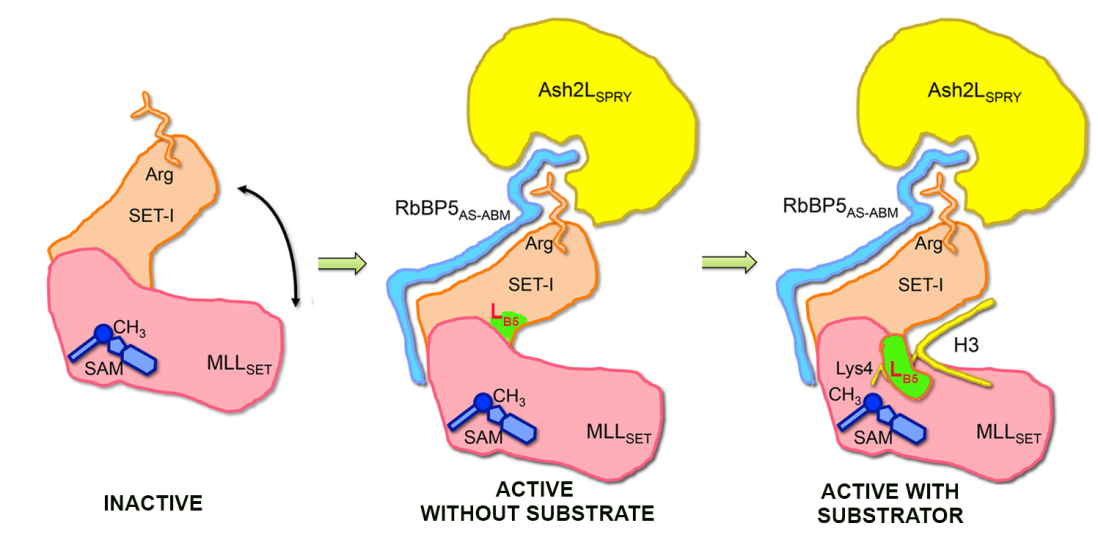
In 2016, our lab collaborated with LEI Ming and LI Guohui to elucidate the molecular mechanism by which the RBBP5-ASH2L heterodimer binds to and activates MLL family proteins. By solving the crystal structures of apo MLL1, MLL3, MLL1-ASH2L-RBBP5, and MLL3-ASH2L-RBBP5, and through molecular dynamics simulations, we demonstrated that the ASH2LSPRY domain and the RBBP5AS+ABM fragment (AS, activation segment; ABM, ASH2L-Binding-Motif) directly binds MLLSET domain and constrains the flexibility of the SET-I motif of MLLSET. The stabilization of MLL by RBBP5-ASH2L leads to better cofactor binding and substrate recognition, thereby enhancing methyltransferase activity (Nature 2016).
https://www.nature.com/articles/nature16952
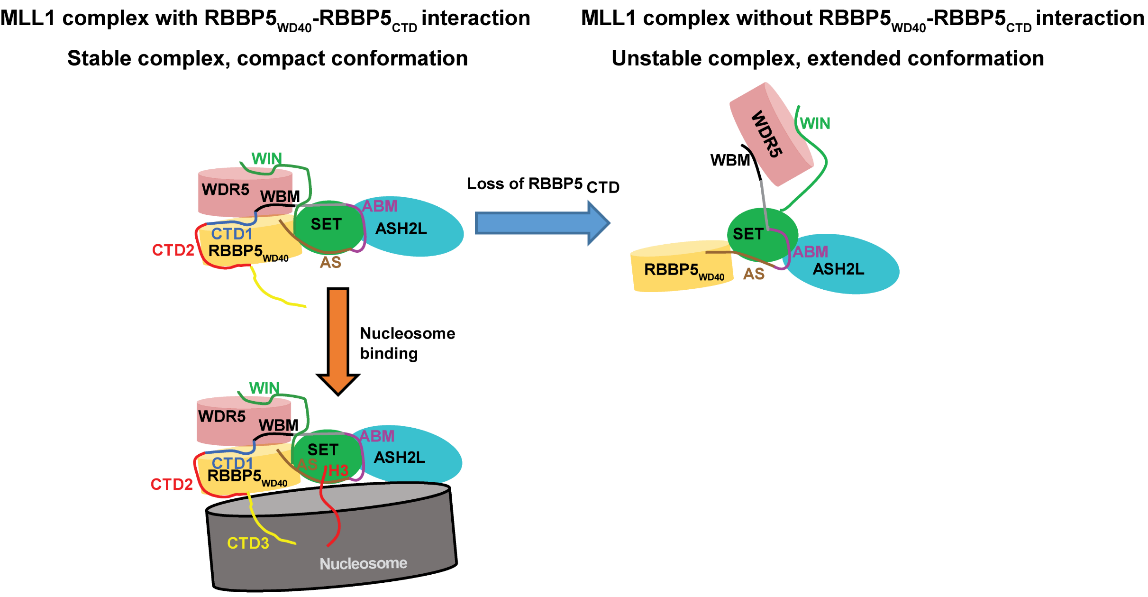
In 2019, we examined the role of the full-length RBBP5 in complex assembly and nucleosome recognition. We identified an internal interaction of RBBP5 important for the optimal activity of the MLL1 complex. The direct interaction between two C-terminal motifs(CTD1,CTD2) and the N-terminal WD40 domain of RBBP5 leads to a more compact conformation of the MLL1 complex, thereby enhancing its methyltransferase activity. We also discovered a vertebrate-specific motif (CTD3) in RBBP5 that contributes to nucleosome recognition (Nucleic Acids Research 2019).
https://academic.oup.com/nar/article/47/19/10426/5572575
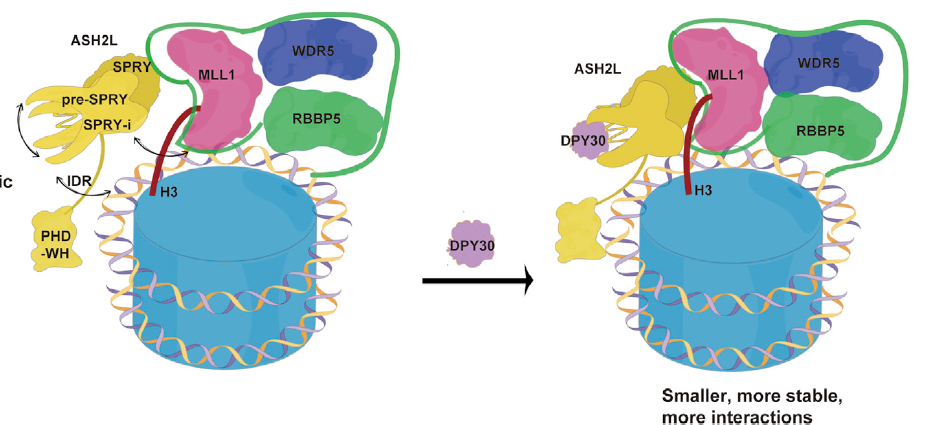
In 2022, we elucidated the dual mechanism by which DPY30 enhances the enzymatic activity of the MLL family complex. DPY30 stabilizes ASH2L by reducing the structural flexibility and increasing the thermal stability of ASH2L. DPY30-stabilized ASH2L has an increased affinity for RBBP5, promoting the formation of a more compact and stable MLL complex. Moreover, DPY30-stabilized ASH2L gains the additional interface for nucleosomal DNA and histone H3, thus greatly enhancing the methyltransferase activity of the MLL complex on nucleosomes (iScience 2022).
https://www.cell.com/iscience/fulltext/S2589-0042(22)01220-2
In 2022, we collaborated to reveal the structural basis for product specificities of MLL family methyltransferases. The highly similar MLL family proteins have different catalytic ability to generate mono-, di-, or tri-methylated products, termed product specificity. Although the product specificities of MLL family methyltransferases have been extensively studied in vitro and in vivo, the molecular mechanisms remain poorly understood. We first developed three quantitative methods (Methyl-Quant WB, Methyl-Quant LC-MS/MS, and Methyl-Quant MALDI-TOF MS) to define the product specificity of histone methyltransferases and identified the specific products for different MLL family members. Furthermore, we found that the dynamics of two critical tyrosine residues (the F/Y switch) in the active site determine the product specificity of MLL family proteins.Distinct interaction networks within the SET domain result in differential dynamics of the F/Y switch across MLL family members, thus fine-tuning the product specificity (Molecular Cell 2022).
https://doi.org/10.1016/j.molcel.2022.08.022

Regulation of Histone Acylation
Lysine benzoylation (Kbz) is a recently discovered post-translational modification associated with active transcription in mammalian cells. However, whether histone benzoylation is widespread across species and which proteins are responsible for maintaining and interpreting Kbz remain elusive. In Nature Communications 2022, we systematically characterized writer, eraser, and reader proteins of histone Kbz in S. cerevisiae using proteomic, genetic, and biochemical approaches. The molecular mechanisms of Kbz recognition by an eraser (Hst2) and by three readers (Taf14YEATS, Sas5YEATS, and Sth1Bromo) were revealed by structural studies. Beyond the conserved histone benzoylation, whole-cell proteomic profiling of Kbz modification expanded the protein substrates containing Kbz to a wide range of proteins, especially proteins involved in ribosome biogenesis and metabolic processes. These results lay the foundation for dissecting the roles of lysine benzoylation in diverse cellular processes (Nature Communications 2022).

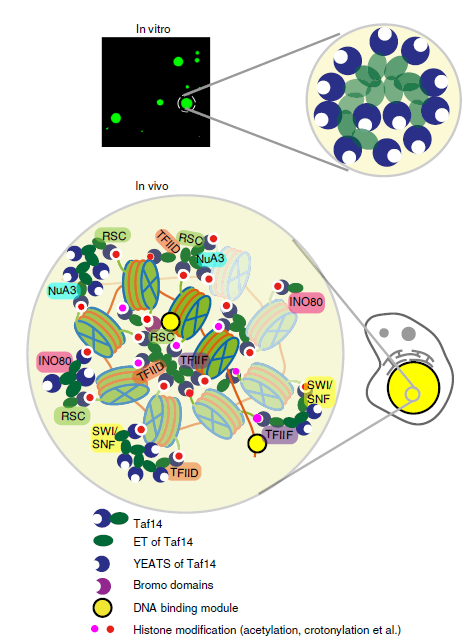
We also explored how histone acylation is linked to other epigenetic and transcription machineries to orchestrate gene expression. S. cerevisiae TBP-associated factor 14 (Taf14) is a transcriptional regulator and contains a YEATS domain that preferentially recognizes acylated H3K9 (acetylation and crotonylation) H3K9. This histone acylation reader protein interacts with more than seven transcriptionally relevant complexes, but how Taf14 associates with these complexes to coordinate their cellular functions in yeast remains elusive. In Nature Communications 2020, through structural and biochemical studies, we demonstrate that the extra-terminal (ET) domain of Taf14 recognizes a common motif in multiple transcriptional coactivator proteins from diverse nuclear complexes. Moreover, we show that such partner binding promotes the liquid-liquid phase separation (LLPS) of Taf14. Thus, beyond identifying the molecular mechanism by which Taf14 associates with different chromatin regulating complexes, our study suggests that Taf14 may function as a versatile nuclear hub to spatiotemporally regulate transcription (Nature Communications 2020).
https://www.nature.com/articles/s41467-020-18021-7
HETEROCHROMATINS
Our lab is interested in the assembly and regulatory mechanisms of heterochromatins, particularly focusing on telomeres. Our research aims to uncover the specific molecular mechanisms by which key telomere-binding proteins and epigenetic factors maintain heterochromatin integrity and regulate heterochromatin localization.
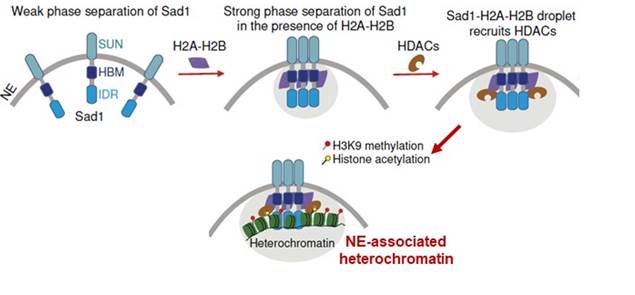
Elucidate How Sad1 Mediates Heterochromatin Assembly and localization
SUN family proteins are highly conserved inner nuclear membrane
proteins and are parts of the LINC complex, linking the nuclear skeleton to the
cytoskeleton and playing essential roles in mechanotransduction. However, the role of
SUN family proteins in heterochromatin regulation remains elusive. Our recent work
reveals that the SUN family protein Sad1 in Schizosaccharomyces pombe interacts
with histones H2A-H2B and plays a crucial role in heterochromatin localization and
silencing. Histones H2A-H2B greatly promote the interaction between Sad1 and histone
deacetylases (including Clr3 and Sir2), ensuring the hypoacetylation state of
heterochromatins. Additionally, H2A-H2B promote the phase separation of Sad1 at the
nuclear membrane, thereby enriching various heterochromatin factors to mediate
heterochromatin formation and NE-localization (Nature Communications
2024).
https://www.nature.com/articles/s41467-024-48418-7
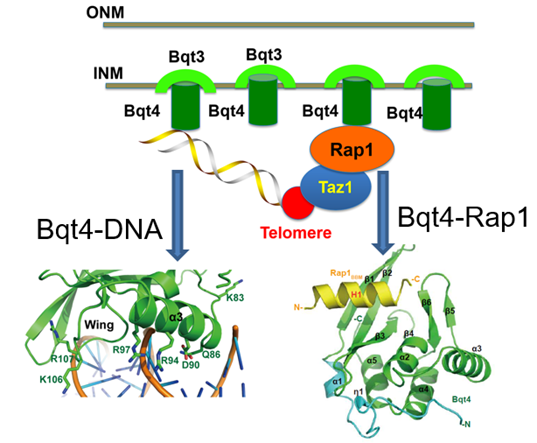
Elucidation of the Molecular Mechanism by Which Bqt4 Regulates Telomere Localization
In eukaryotes, many heterochromatin loci associate with the nuclear envelope (NE). The telomere is the best-characterized heterochromatin region associated with the nuclear envelope. The connection between telomeres and NE is critical for maintaining chromatin homeostasis and proper cell division, but the molecular details of this connection remain unclear. S. pombe inner-nuclear-membrane protein Bqt4 is a crucial molecule for the telomere-NE association. Two of our studies collectively reveal how Bqt4 interact with DNA and other proteins to help telomere anchoring to the NE.
In the paper published in Structure, we identified a novel DNA-binding activity of Bqt4. This DNA-binding activity of Bqt4 may prime the chromosome onto the NE and promote telomere-NE association. In the paper published in Nucleic Acids Research, we determined the crystal structure of the Bqt4-Rap1 complex and revealed that Bqt4-Rap1 interaction is important for the telomere-NE association and meiosis progression. Phosphorylation of Rap1 acted as a switch to regulate the interaction between Bqt4 and Rap1. In summary, these two studies indicate that Bqt4 is an important platform protein with multiple binding partners ensuring proper telomere association with the NE.
https://academic.oup.com/nar/article/47/3/1573/5193554
https://www.cell.com/structure/fulltext/S0969-2126(18)30371-X
Elucidation of the Structure and Function of the Telomere Protein Complex
In mammalian cells, telomere-binding proteins include TRF1, TRF2, POT1, TPP1, TIN2, and
RAP1, which together form a protective complex known as Shelterin. Shelterin regulates
telomere length homeostasis and prevents erroneous DNA damage repair. Our lab has
previously determined the structures of several Shelterin sub-complexes, including
TRF1-TIN2, TRF2-TIN2, and RAP1-TRF2 complexes (Science 2008; NSMB
2012). Recently, we determined the crystal structure of an important "bridge"
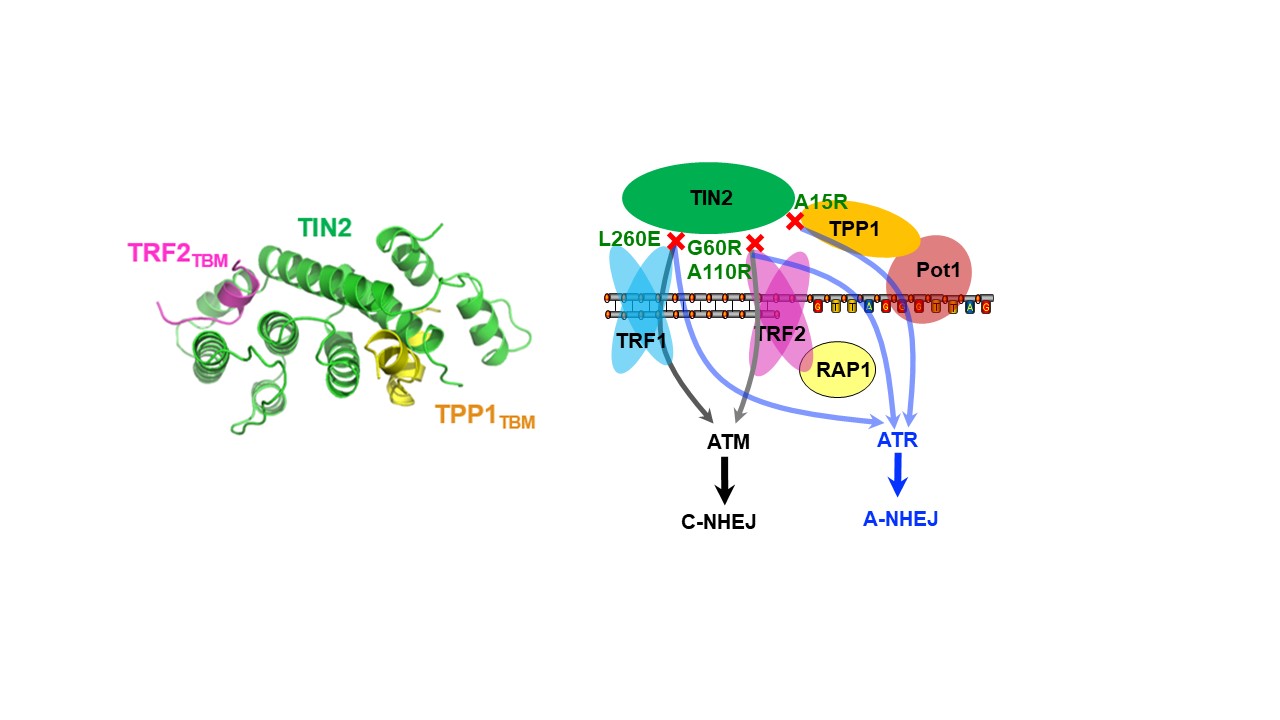
subcomplex in Shelterin—TIN2-TPP1-TRF2 complexes. Surprisingly, TIN2 contains a
telomeric repeat factor homology (TRFH)-like domain that functions as a protein-protein
interaction platform. The structural and biochemical analyses uncovered the mechanism by
which TIN2 simultaneously binds to TRF2 and TPP1 to mediate cooperative assembly of the
telomere complex. We also defined the precise roles of proteins such as TIN2, TRF1,
TRF2, and TPP1 in suppressing ATM and ATR damage signals, as well as in inhibiting the
NHEJ (non-homologous end joining) and HR (homologous recombination) pathways (Cell
Research 2017).
https://www.nature.com/articles/cr2017144